Understanding how levodopa works in Parkinson's (part 2)
This week we are getting pretty technical. I'll rely on you to tell me if this week's post is useful post or if I've gone too far. Send me an email to tell me that I should not shy away from presenting technical information, with the risk that I don't do so clearly enough, or that this is too much. This will help in particular in future posts as we consider the potential for GLP-1 agonist therapy for Parkinson's.
More Lessons from Pharmacology
Mechanism of Action of Levodopa
Let's first consider how levodopa (the name of L-DOPA, the dopamine precursor, when used as a drug) works (focusing on its effects in the brain) when Parkinson's is not present (which is the discipline of pharmacology).
The figures below show the relationship between two communicating neurons (brain cells). Communication from the first to the second neuron is necessary to initiate, continue and control movement. In each figure, the presynaptic neuron is towards the top where the signal is initiated. Below is the postsynaptic neuron, where the signal is received. The signal must be strong enough to activate the second neuron and allow for control of movement. The space between them is called the synapse and is filled by cerebrospinal fluid. Dopamine needs to cross the synapse - from presynaptic to postsynaptic neuron - for successful communication between these neurons.
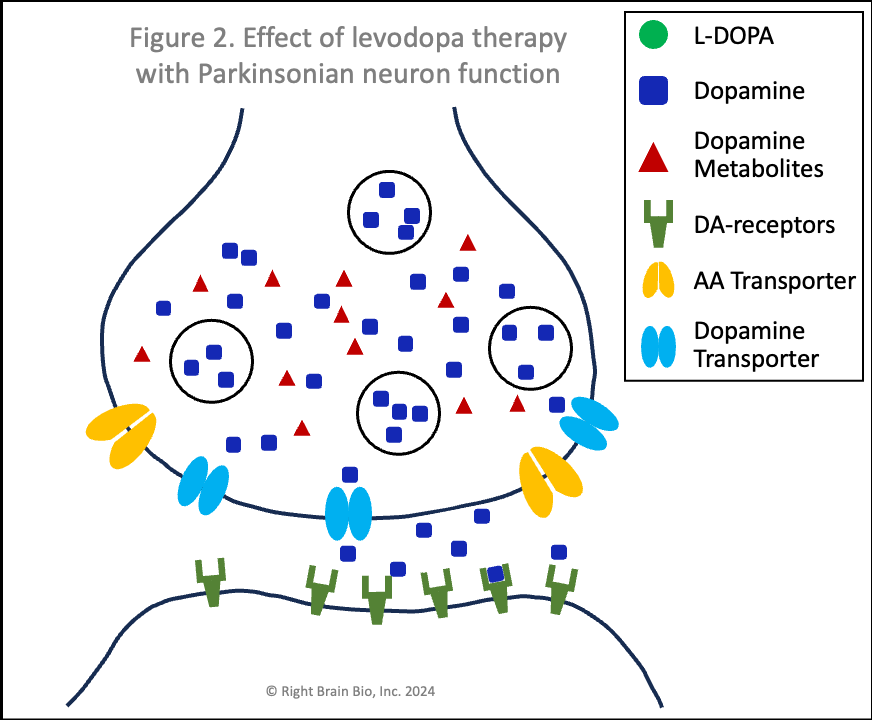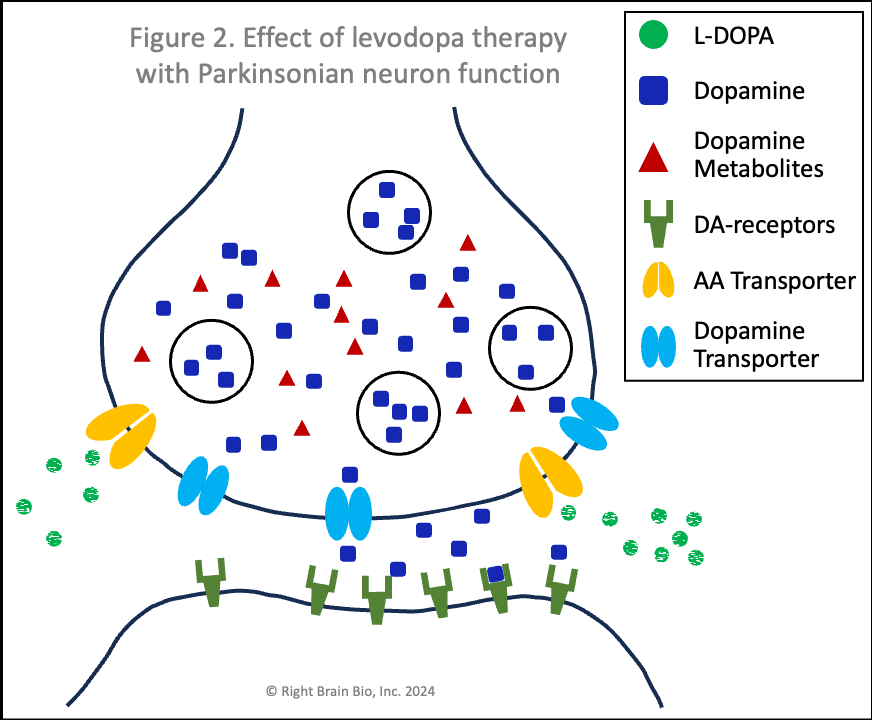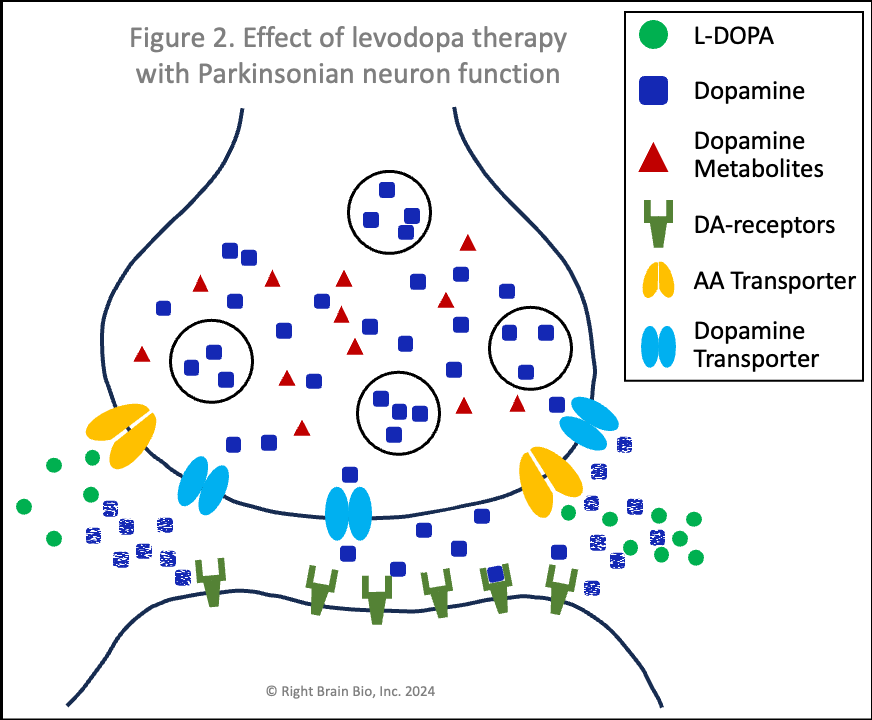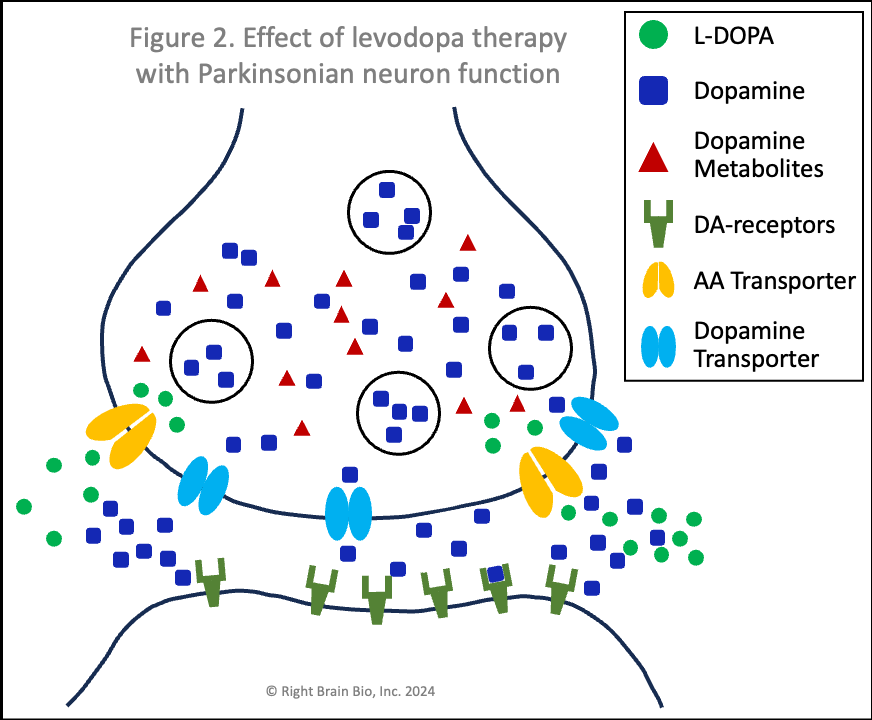
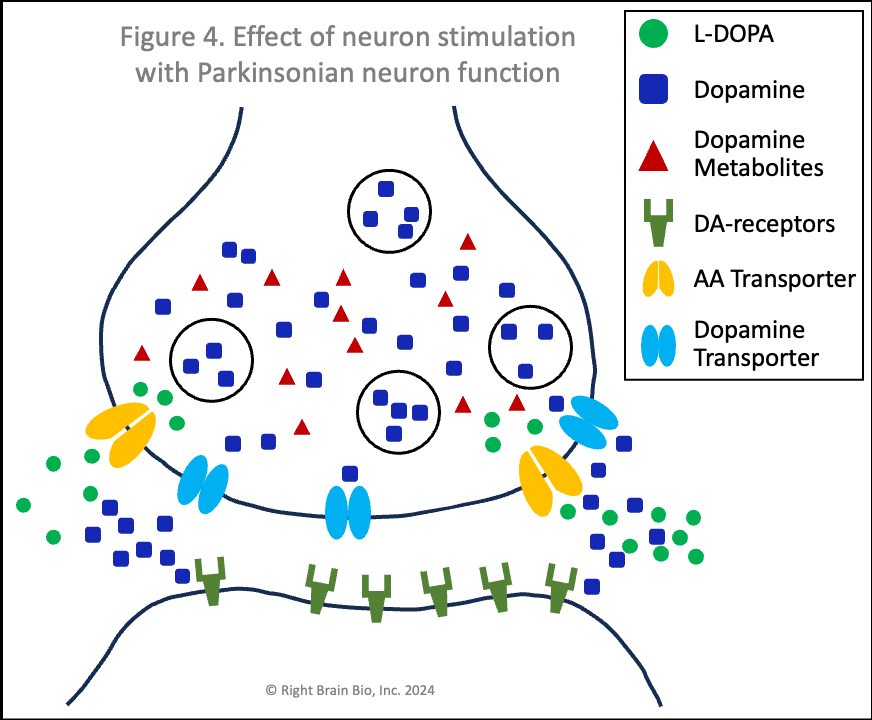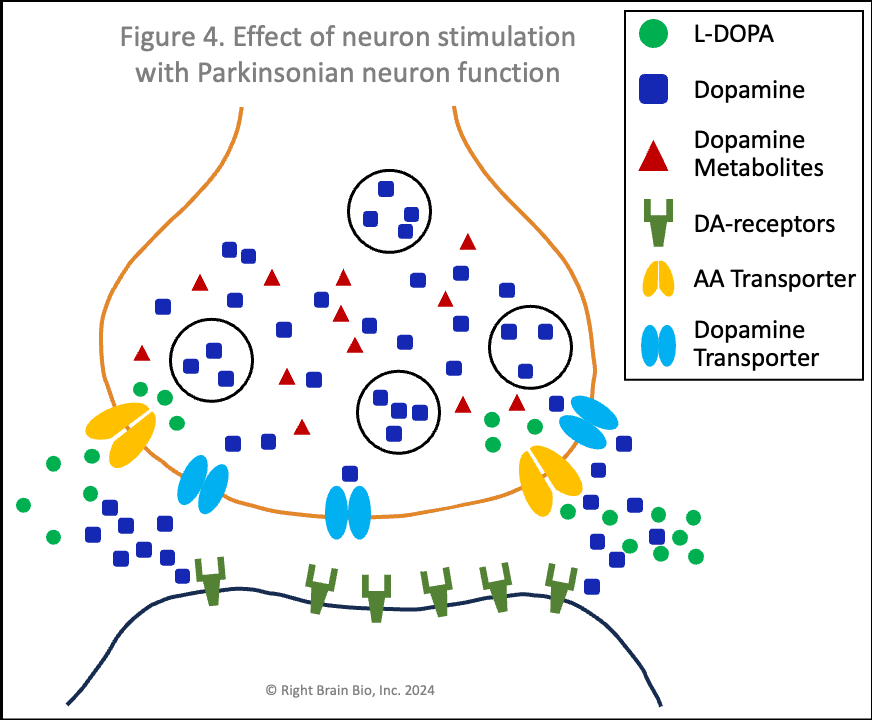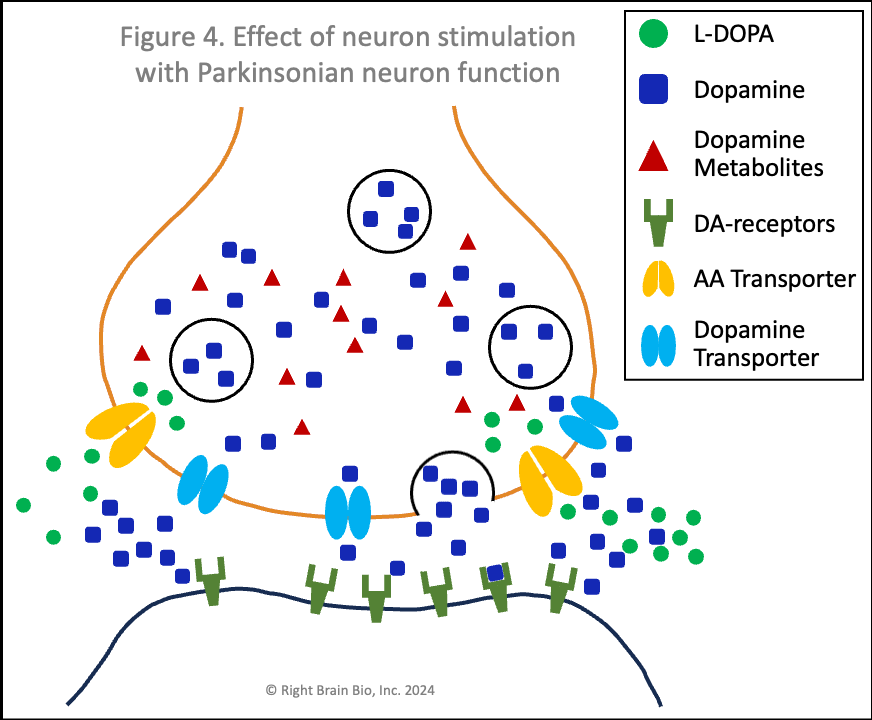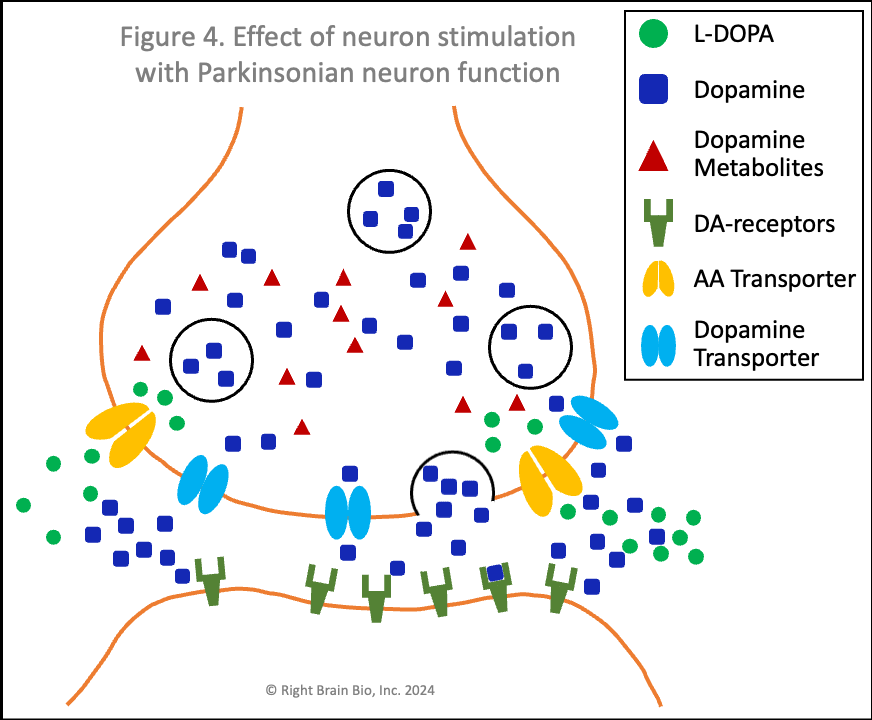
Figures 1 and 2 show the effect of levodopa therapy in those without (Fig 1) and with (Fig 2) Parkinson's. In either case, the drug crosses into the central nervous system and diffuses throughout the brain including into the synapses as shown here with the levodopa appearing as green circles.
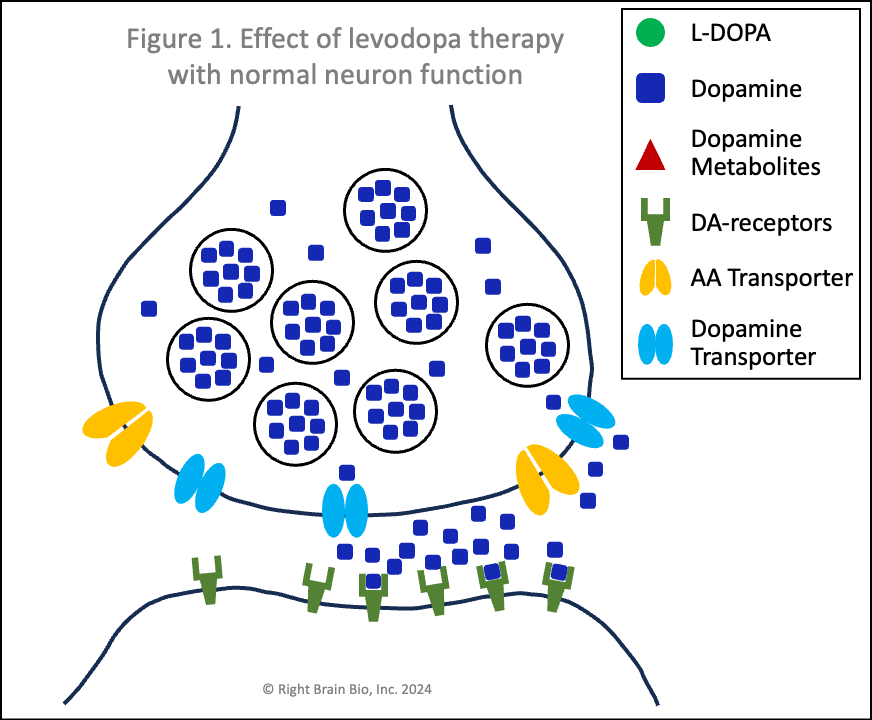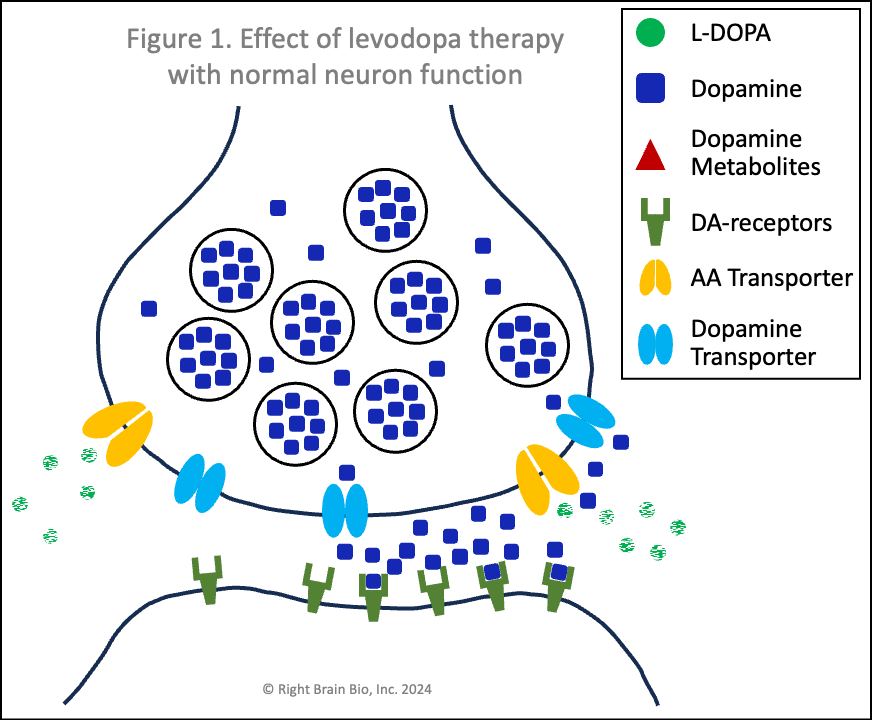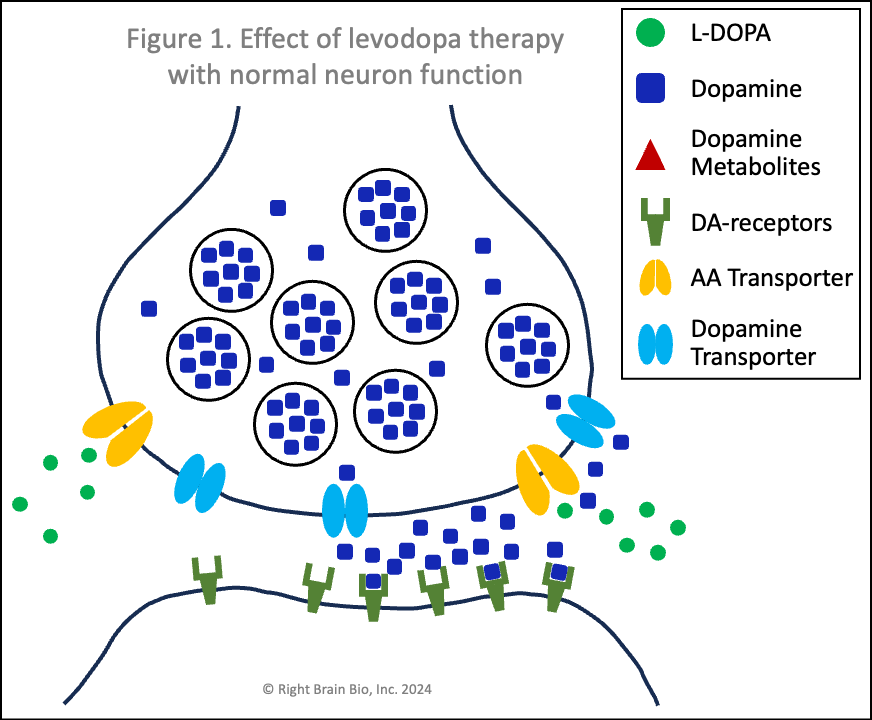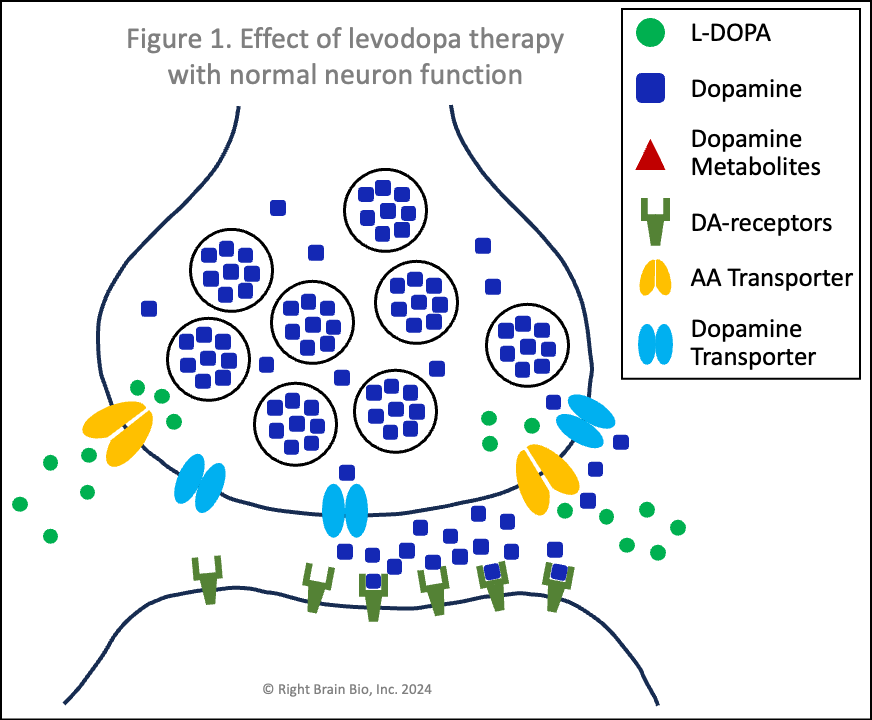
In Figure 1, the levodopa is taken up by the presynaptic neurons through amino acid transport channels (called AA transporters shown in light orange). The levodopa inside the presynaptic neuron is converted rapidly to dopamine (blue squares) and sequestered inside vesicles (larger blue circles), which prevent the dopamine from being metabolized into toxins.
In Figure 2, the levodopa is partly taken up by the presynaptic neurons through AA transporters. Shown in Figure 2 is that some of the levodopa in the synapse is converted into dopamine (blue squares) outside of the cells. Recent studies show that people with symptomatic or not yet symptomatic Parkinson's can convert levodopa to dopamine outside of cells, which is not possible without Parkinson's. That dopamine can increase the sensitivity of post-synaptic dopamine receptors - the receivers of neuronal communication. I'll explain why that is important in a bit. The dopamine in the synapse ordinarily would be taken up through the dopamine transporters (DAT, shown in light blue), but since that requires energy and the dopaminergic neurons suffer from an energy crisis in Parkinson's, that dopamine largely remains outside the neurons and within the synapses.
Also note how in Figures 2 and 4, the vesicles are fewer in number and less full of dopamine than in Figures 1 and 3. When a signal to initiate or control movement comes from the brain which activates the presynaptic neuron, the vesicles move toward the synapse and release their dopamine into the synapse to communicate with the neuron across the synapse. So with lower number of vesicles and less dopamine within the vesicles, neurons in people with Parkinson's send a weaker signal from the activated presynaptic neuron to the postsynaptic neuron. If the signal is small enough, it won't be sufficient to activate the post-synaptic neuron, and there will be loss of control of movement.
Figures 3 and 4 show what happens without or with Parkinson's disease, and each starts with the presynaptic neuron being activated - shown as the neuron outline flashing from black to orange, and therefore, wanting to communicate with the postsynaptic neuron.
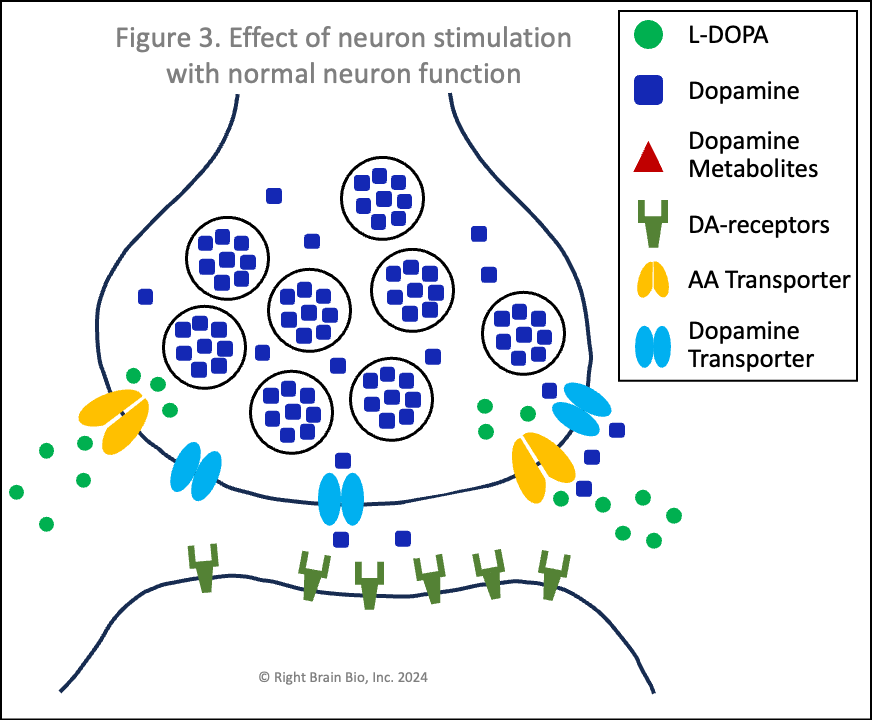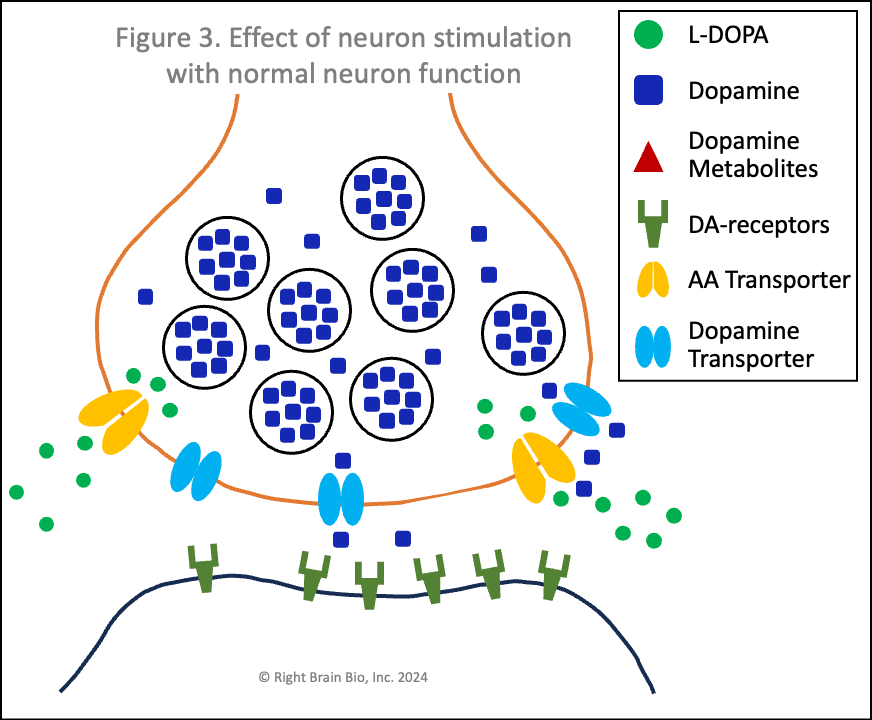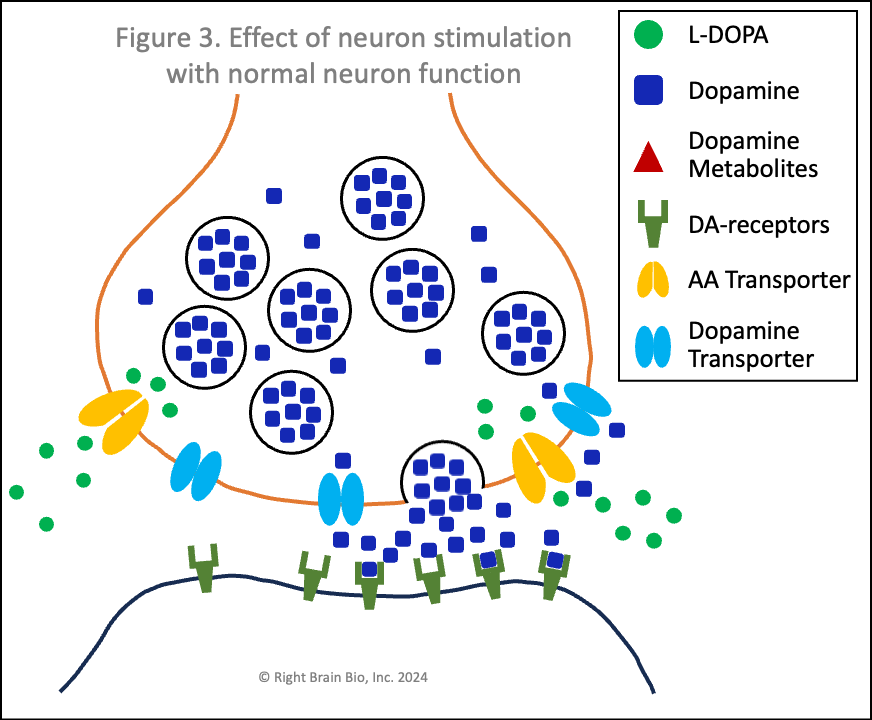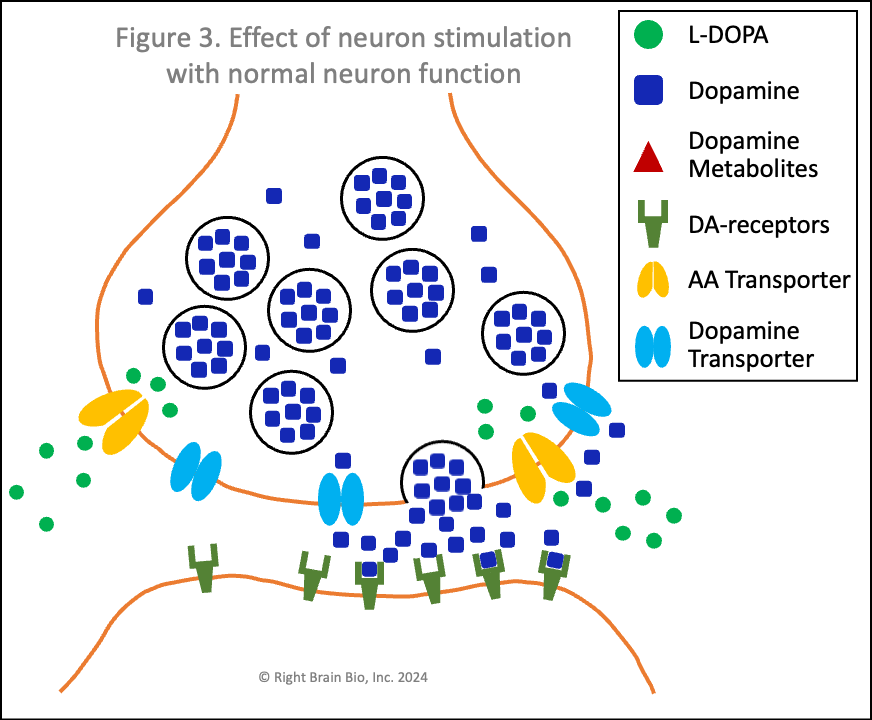
Vesicles (larger circles) move towards the synapse to prepare for releasing their dopamine (blue squares). The movement and the release are both active processes. That means that neither works very well in Parkinson's (Figure 4) compared to the non-Parkinsonian state (Figure 3) owing to the energy crisis within these brain cells.
Under normal conditions (Figure 3), the flood of dopamine (blue squares) into the synapse leads to dopamine binding to the postsynaptic dopamine receptors (olive-green sticks) in sufficient amount to activate the postsynaptic neuron as shown by its flashing black to orange.
In Parkinson's (Figure 4), the amount of dopamine released is less and the amount binding to postsynaptic receptors is less. In people with worse Parkinson's, the amount released that binds to receptors may not be enough to activate the post-synaptic neuron, which would prevent normal control of movement. The situation shown here in Figure 4 does result in enough stimulation to activate the postsynaptic neuron (shown by outline flashing black to orange). This happens because of levodopa therapy, as the extra dopamine formed from levodopa outside the cell raises the amount of dopamine in the synapse, which makes the postsynaptic receptor more sensitive.
I referred to this earlier. Increasing the amount of dopamine in the synapse will increase the sensitivity of the postsynaptic receptor. This relationship is shown for many receptors inside the body that are of the same type, called G-protein coupled receptors.
My hypothesis is that this is how levodopa actually works to restore movement. Other proposed mechanisms require the diseased neurons that suffer from an energy crisis to somehow find energy when levodopa is administered to enable their cells to function normally. There is no evidence this happens. This is why I believe that levodopa works for people with Parkinson's by sensitizing these receptors, a mechanism that does not require the energy that Parkinsonian neurons lack.
Remember last week when I discussed the ranges of treatments with a disconnect between immediate and long-term effects? Studies of G-protein coupled receptors in people from organs other than the brain show that this increased sensitization of receptors happens quickly, and also that over time, it wears off and these receptors become less sensitive to dopaminergic stimulation (called desensitization). This phenomenon explains why many people feel a profound benefit of levodopa in the first days, weeks or months of use which goes away over time, typically requiring an increase in doses, which should overcome the desensitization for some time, before wearing off again.
This Week's Message
My hypothesis of how levodopa works (and often, does not work) is based on integrating several known factors: (1) that immediate and long-term effects of therapies can be different as discussed in last week's newsletter, (2) that the dopaminergic neurons suffer from an energy crisis in Parkinson's that limits many cellular activities as a result of loss of mitochondrial number and function, (3) that G-protein coupled receptors behave in a consistent and predictable manner particularly as discussed above for sensitizing and desensitizing these receptors and (4) that levodopa works for weeks or months in most people, but eventually, the benefits wear off.
L-DOPA therapy is a critically important part of treating Parkinson's owing to its benefit on movement. However, there are no data showing that L-DOPA is a long-term answer. Its proposed mechanism of action is based on how neurons work in the absence of Parkinson's, and with the knowledge of how neuronal dysfunction with onset of Parkinson's (even prodromal), different models of disease and treatment must be considered. Doing so will allow us to identify new and hopefully improved ways to treat the disease.
Share This|
Sign up at: ParkinsonsDisease.blog |
About Jonathan Sackner-Bernstein, MD
Dr. Sackner-Bernstein shares his pursuit of conquering Parkinson's, using expertise developed as Columbia University faculty, FDA senior official, DARPA insider and witness to the toll of PD.
Dr. S-B’s Linkedin page
RightBrainBio, Inc. was incorporated in 2022 to develope dopamine reduction therapy for people with Parkinson's.
