Dyskinesia part 2: learning from clinical data
Several weeks ago, I shared my desire to understand dyskinesia. This week I share what I've learned so far.
As I described, dyskinesia is thought to be due to long-term treatment with levodopa where the levodopa is administered intermittently, with dyskinesia related to levels of dopamine getting too high. In that context, several different versions of levodopa were developed, including pills that release the drug more slowly and a gel form that is infused at a constant rate through a catheter into the intestine. And recently a form was studied that is infused through a catheter that is inserted through the skin into the subcutaneous space (meaning under the skin).
By evaluating how these different forms work, perhaps we can learn more about the cause(s) of levodopa-induced-dyskinesia, and then be able to see a way to prevent or reverse it.
I like to start with the basics - stuff that is relatively simple. This is typically referred to by scientists as the evaluation of First Principles, the foundational assumptions, data and/or observations. In this case, let's see if there are data that show dyskinesia is related to peaking drug levels. And my preferred approach is to start by identifying controlled scientific studies.
What is a controlled study?
When I speak of a controlled study, it means that there is a comparison between different treatments, typically one being a standard or typical treatment, which serves as the control (or a comparator). The other treatment(s) are compared to the control to learn if there are differences.
A controlled clinical study is called randomized and blinded when the people enrolled in a clinical trial are randomly assigned to receive one or another treatment and neither they nor their doctors know which. This could mean one group gets a placebo or when the study seeks to compare two possible therapies, an active control. Such an example of the latter relevant to understanding dyskinesias in people with Parkinson's would be a comparison between two ways of using levodopa as a therapy. One group would get the old-fashioned pills with levodopa-carbidopa - so-called immediate release, which get into the brain quickly, produce high levels of dopamine and then almost as quickly lead to a drop in the brain levels of dopamine towards where they started. The other would get an infusion of levodopa-carbidopa into their intestines, so that the levels of dopamine in the brain stay the same throughout the day.
In this kind of trial, all participants get a catheter inserted into their intestine with half getting the drug and half a placebo (that has no levodopa-carbidopa) and all participants also get pills, with half containing active drug and the other half a placebo. This kind of clinical trial is demanding on people who enroll since they all must undergo implantation of an intestinal catheter and wear a pump on their waist during the day.
So a controlled, randomized and blinded study produces data least subject to bias and therefore should be the starting point for any analysis (if any have been performed).
Testing the hypothesis that levodopa-induced-dyskinesia is caused by rapid peaks in the brain levels of dopamine.
If levodopa-induced-dyskinesia were simply a function of brain levels of dopamine getting too high, then there should be a relationship between the time when the levels are highest and when the dyskinesias are the worst. This is the kind of simple way to understand this First Principle.
Here's how that could work. The data below are from a study that was not blinded which compared the blood levels of levodopa from either of the two versions of the drug. I added the arrows, as these are the times when one could look for more dyskinesia or worse dyskinesia if the cause were as simple as having peak levels that are too high. Spoiler alert: it's going to be more complicated than that. But this kind of analysis would be valuable to prove the high peaks are not the only reason for dyskinesia when taking levodopa.
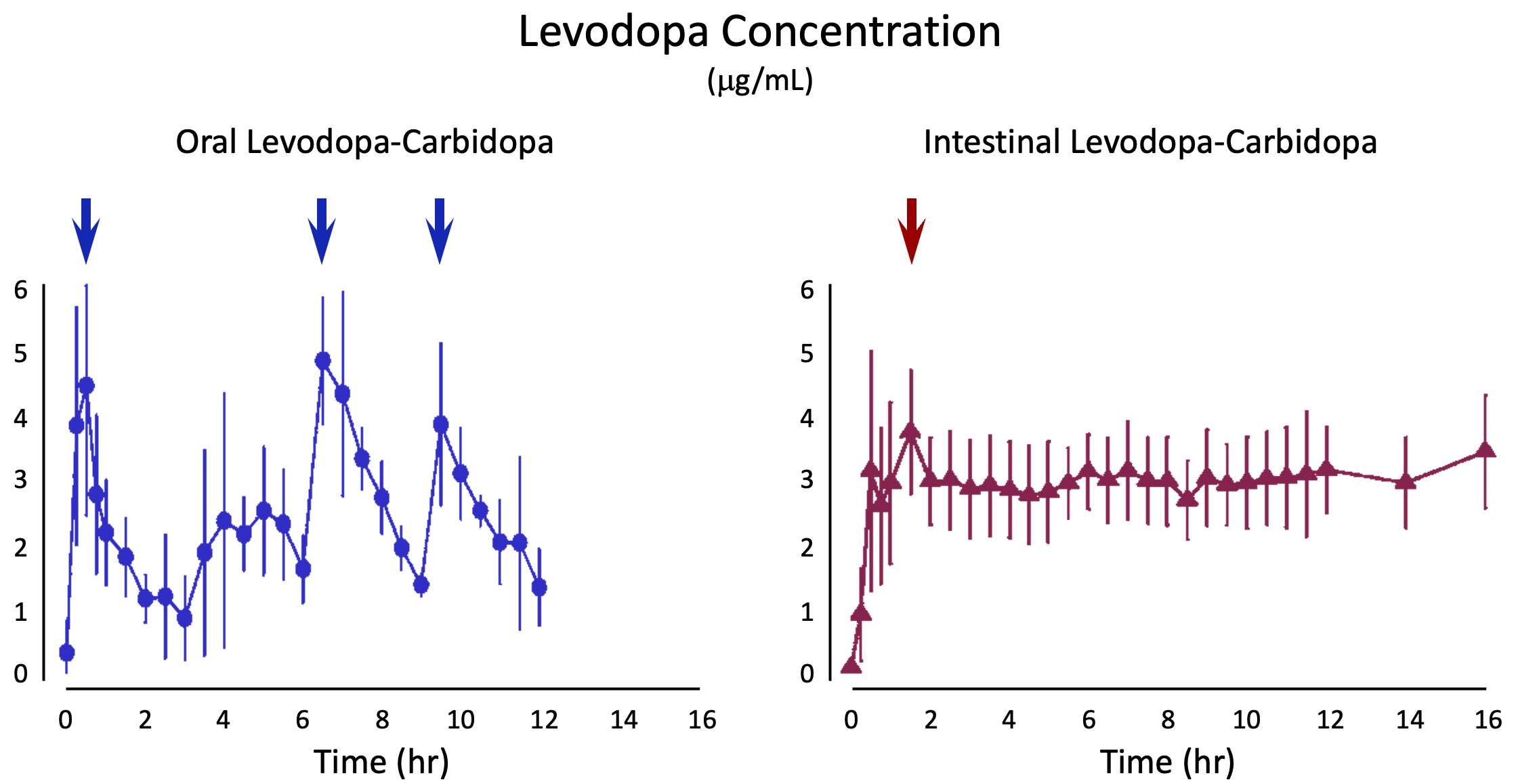
I found one illustrative randomized, blinded, controlled, peer-reviewed publication comparing immediate release with constant release, where the latter was levodopa infused by a pump through a catheter into the intestines. Dr. Olanow and colleagues performed a study sponsored by Abbvie (which sells the intestinal version of the drug). They measured the blood levels of levodopa, which is a good approximation for the changes in brain levels as levodopa crosses from the circulation into the brain readily. It's primary purpose was to determine whether there was a difference between the two versions of drug in "off" time, which is the time when a person is not experiencing benefit.
For those unfamiliar, "off" time is when the medicine doesn't seem to be working. Another way of thinking about it is that it is the time when a person can tell the benefit is "wearing off." One thing that bothers me about how this term is used is that a person can't really define "off" without experiencing "on" time. It's all relative. So how should people rate the condition at a particular moment if their symptoms do not respond to medicines? This is a tangent that we should put on the back-burner, though it shows how complicated it can be to design and run a clinical trial.
This study by Olanow and colleagues randomized 71 people with advanced Parkinson's who suffered "off" periods and who also experienced dyskinesia for about 3-4 hours a day. While the study showed that the intestinal infusion significantly reduced "off" time about 2 hours more each day (starting from a baseline of 6-7 hours of "off" time each day), the rate of people in the study experiencing dyskinesia was not different. In the intestinal infusion treatment group, dyskinesia was still present in 14% while in the oral dose treatment group, dyskinesia was present in 12%.
Despite eliminating peak levels, people still had dyskinesia, although the study did demonstrate that the severity of dyskinesia was meaningfully less with intestinal infusion.
This study does not prove that levodopa-induced-dyskinesia (or as some call it, levodopa-related-dyskinesia) is caused by excessively high peak levels of the drug. And it does not show that going through the procedures to get intestinal infusion is worth it if the goal is to get rid of dyskinesia, though it will reduce severity.
While I don't understand yet the cause - or the optimal way to treat - levodopa-induced-dyskinesia, I can share two inferences. First, constant infusion of levodopa is reasonable to pursue if the goal is to reduce "off" time or reduce the severity of dyskinesia, but not if the goal is to eliminate dyskinesia. Second, the cause (and therefore possible treatments) of levodopa-induced-dyskinesia is not as simple as excessively high peaks. And while I did not discuss here, I don't think the data support the cause as being related to excessively low valleys in drug levels either. It's just more complicated than that.
To me, the study of dyskinesia has been frustrating and left me feeling empty. I expected to learn something that would be actionable. Perhaps I still will, but if there were something, I expect we'd be able to find out about it pretty easily. Hopefully, I'll learn something that will make me more optimistic about dyskinesia. And when I do, I will share it.
Great feedback on the podcast request. Thanks to each of you who provided a recommendation and/or idea. I am putting together pitches for several of those suggested.
Share This
|
Sign up at: ParkinsonsDisease.blog |
About Jonathan Sackner-Bernstein, MD
Dr. Sackner-Bernstein shares his pursuit of conquering Parkinson's, using expertise developed as Columbia University faculty, FDA senior official, DARPA insider and witness to the toll of PD.
Dr. S-B’s Linkedin page
RightBrainBio, Inc. was incorporated in 2022 to develope dopamine reduction therapy for people with Parkinson's.
